Why do you need lye to make soap? (FAQS)
Learn why you need lye to make soap and how to handle it safely.

Our great great grandmothers made soap without having to use a caustic chemical, so why do we?
The short answer is that our grandmothers manufactured their own caustic chemical of sorts, called potash, by combining hardwood ashes and water.
This created an extremely strong alkaline substance that was considered ready when it would dissolve a chicken feather. The caustic wood ash solution was then mixed with fat rendered from butchered animals and boiled over an outdoor fire for many hours until a soft soap was formed.
While this method resulted in a truly natural soap, it was also difficult to control the quality of the final product.
These days, we have standardized substitutes to replace that wood ash solution:
- Sodium Hydroxide (NaOH) – also called caustic soda or lye; creates solid bars of soap
- Potassium Hydroxide (KOH) – is used to make liquid soaps
Using these types of lye and precise measurements, we’re able to consistently produce batch after batch of gentle soap.

What happens if you leave the lye out of a recipe?
Without lye, the oils in your recipe would stay oils. Nothing would happen to them.
A chemical change involving lye must happen in order to create soap.
Can you use glycerin instead of lye?
Some people mistakenly believe that glycerin can be used instead of lye. Glycerin (a humectant that’s good for your skin) is actually a byproduct of the soapmaking process and will not transform oils into soap.
If you try to use glycerin instead of lye in a soap recipe, it would be sort of like trying to start a fire with a fresh rose petal instead of a match. It just won’t work.
However, you can buy ready made glycerin soap bases (melt and pour soap, which is high in glyerin) if you don’t want to handle the lye part at home. (See my blog post “Making Soap Without Lye (Sort Of)” for more on melt and pour soaps.)

What about organic and natural store-bought soaps? Aren’t they lye free?
All true soaps are made with some form of lye. If not, then they’re detergent based products.
Some, like Dr. Bronner’s soaps, are made simply with just lye, oils and a few natural extras. Others, like Dove, are made with a combination of lye and detergents.
You can tell if a soap is made with lye, by looking for the following clues in the ingredients:
- “sodium tallowate” = beef fat reacted with sodium hydroxide (lye for bar soap)
- “potassium tallowate” = beef fat reacted with potassium hydroxide (lye for liquid or cream soaps)
- “sodium olivate” or “potassium olivate” = olive oil reacted with lye
- “sodium cocoate” or “potassium cocoate” = coconut oil reacted with lye
- “sodium lardate” = pig fat reacted with lye
- “saponified” means that one of more of the oils have been reacted with some form of lye
- example: “saponified organic coconut oil” = coconut oil reacted with sodium hydroxide (bar soap) or potassium hydroxide (liquid soap)
How can such a strong substance create a mild and gentle soap?
You need lye to make soap, even natural ones, but it just doesn’t make sense at first thought, does it?
Hopefully, this section will give a better idea of how that happens.
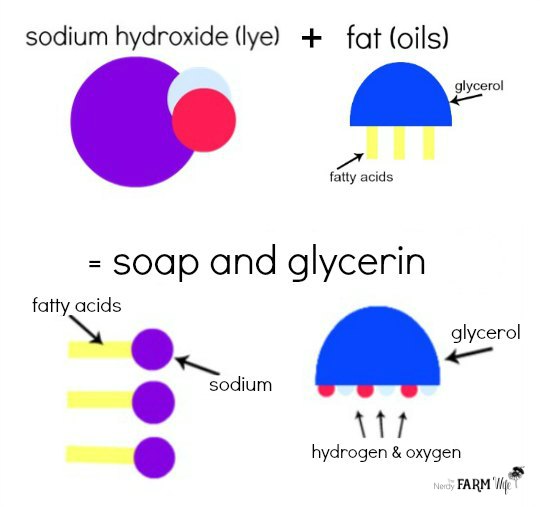
With apologies to real chemists everywhere, below is a super-simplified illustration of how lye (sodium hydroxide) and oils/fats (glycerol & fatty acids) combine to make soap. In the presence of heat and water, the “fatty acids” bond with the “sodium” part of sodium hydroxide and form soap. The “glycerol” and “hydroxide” parts form glycerin, which is great for your skin. No lye (sodium hydroxide) is left in the final soap!
Isn’t chemistry amazing?!
Are you afraid of lye?
If so, you’re not alone; many people are. I can relate, because I was the same way! When I first started making soap, I was terrified at the thought of using lye and had my husband handle that part. After making my first successful batch of soap though, I realized…. lye isn’t that scary after all!
If you’ve ever safely worked with bleach or another strong household chemical, you should be able to handle lye just fine. The main thing to remember is that it’s a caustic substance. Just like bleach, you don’t want to drink it or get it in your eyes or on your skin. The fumes aren’t good to breathe in either.
There’s no need to fear lye, but it does deserve a good deal of respect and care when handling.
I’ll share a list of safety tips below.

Lye Safety Tips
1. Always wear gloves and goggles to protect your hands and eyes when working with lye and raw soap. (Raw soap is less than 24 hours old.)
2. If you get lye solution or raw soap on your skin, rinse thoroughly with lots of cool water. Don’t worry that a brief exposure will eat your skin off or anything drastic like that; it feels similar to a stinging sunburn until it’s rinsed off, but you do want to rinse it off quickly. While some soapers like to use vinegar in an effort to neutralize lye, the majority of safety experts recommend plain water. If lye solution gets in your eyes, rinse repeatedly with cool water and call your eye doctor for an emergency visit. (Always wear your goggles though, and this should not happen!)
3. Always add lye to water, and not the other way around. You can remember this by thinking about snow flakes falling on a lake. (Flakes of lye, falling on the water.) If you mix up the order (and I have before), it won’t blow up or anything, but could bubble up and overflow out of a small container. In my case, nothing out of the ordinary happened, but still, it’s good to go by the recommended order. I also suggest mixing the lye solution in your kitchen sink to contain any spills.
4. When lye first meets water, it gets really hot, really fast. Use room temperature or cooler water/liquids, since warm liquids can overheat and overflow. Don’t use glass for this part because it could shatter. The lye solution will also give off strong fumes for about 30 seconds or so. Don’t breathe these in and be sure to work in an area with good air flow, or even outside. If you have breathing problems or are concerned about the fumes, wear a respirator mask.
5. Don’t leave bottles of lye, or containers of lye solution, around where pets, children, or other members of the household could accidentally spill or drink them. Drinking lye can be fatal to both humans and pets. Don’t make soap with pets or small children around.
* Remember, these safety tips are in place to help prevent worst-case scenarios. People make soap all over the world, every day, without incident. Work carefully and responsibly at all times.
Want to learn more about making your own soap?
For natural soap recipes, tutorials and inspiration, be sure to check out my Natural Soap Making Ebook Collection:

The information on this site is for idea-sharing only. While this site does its best to provide useful information, any reliance you place on such information is strictly at your own risk and not a substitute for medical, legal, or any other professional advice of any kind.




Great explanation, I get tgis question a lot. I am curious to try the potash method though.
Hi Liz! I’m glad you enjoyed the article! I’ve always wanted to experiment with making lye from wood ashes too; it’s on my things-to-try-one-day list!
great information in this post – thank you very much
Hi Kristin! Happy you found the post helpful!
Hi can I substitute sodium hydroxide with sodium Lactact.
Hi Jacky! No, you sure can’t. Those are two completely different things.
Sodium hydroxide is a caustic substance that turns oils into soap.
Sodium lactate is a salt that helps to harden soap.
More information about why we need lye for making soap is in this article you commented on. :)
in one of the pictures above the soap is being cut with what lookes like thick dental floss. It also looks like it is a press of some sort. where can I find something like this to cut soap? Thanks.
PS very informative site!
Hi Brian! Glad to hear you’re enjoying the site!
That’s a multi wire soap cutter. My husband made that one for me, but you can buy one like it on Etsy:
https://www.etsy.com/search?q=multi%20wire%20soap%20cutter
I have a question. If I need sodium and hydroxide why can’t I use their pure forms instead of lye?
Hi Christopher, Great question! I’m not a trained chemist, so that’s way outside the scope of my knowledge base. There are some tutorials out there on making your own sodium hydroxide, but sounds like it’s not a safe idea:
https://www.scienceforums.net/topic/39475-is-it-practical-to-make-sodium-hydroxide/
This one is the best and in the same time most calming and well presented explanation! This is something i try to explain my clients, the better is to bring them here
Hi Krasimira, I’m happy to hear you like the article! :)
Hello Jan,
I have been following you and making soaps for three years. But, this time, I made a big mistake and used potassium hydroxide instead of sodium hydroxide for my 3 CP batches:((( I would like to ask for your kind advice if I still can save these batches and can use them? I will appreciate for your answer in advance and hope I can save them?
Kind Regards,
Elif
Hi Elif! Oh no! I’m so sorry to hear that happened! I’ve had terrible mixups too – it’s never fun!
What you would have to do is take the recipes and run them through a lye calculator, only using potassium hydroxide instead of sodium hydroxide. That will let you know if they still have a safe lye amount.
For an example soap:
If my recipe was:
20 ounces olive oil
8 ounces coconut oil
3.97 oz sodium hydroxide for a 5% superfat
If I accidentally used 3.97 ounces potassium hydroxide though, then the lye calculator tells me that the superfat is now 39%. Eeek! That would probably be pretty unsalvageable unless you want to do some quick math and rebatch with more diluted potassium hydroxide to make up the difference.
In this case, the soap calculator tells me that I need 6.13 ounces potassium hydroxide to turn this recipe into a 3% superfat. (A high range for liquid soap.)
So you could take 6.13 oz needed – 3.97 already used = 2.16 ounces that you could dilute with 3x as much water and stir into the batch while rebatching in a crockpot.
This would turn it into a liquid soap and you can dilute it with water or water/glycerin as I do in this recipe: https://thenerdyfarmwife.com/dandelion-honey-liquid-soap-recipe/
Thank you very much for the explanation I love it
Hi Omowumi, Thanks for your comment! I’m happy to hear you like the article!
Hello, am planning to start classes for soap making so as I can start a small enterprise for soap production. I love explanation of everything. I would like to learn more.
Hi Tutu! You may enjoy my Soap Tip Tuesdays email series in which I share my best soapmaking tips and articles!
You can sign up here: https://thenerdyfarmwife.com/herbs-in-soap/
Good luck with your upcoming enterprise! :)
I like your soap cutting board better. Is hubby making any more? lol
Hi Michelle! I wish he would, but unfortunately, he says that was his one and only soap cutter project. :)